Adenomyosis is a condition where the tissue that normally lines the inside of the uterus is found within the muscular layer of the uterus. It is common in women who are of childbearing age, and can develop at any age. The most common symptom of adenomyosis is unrelenting pain, throughout the cycle, on the top of the uterus. Severe period pain is common and there may be heavy bleeding.
Adenomyosis can occur alongside endometriosis. Although endometriosis can be found in about 1 in 10 women of reproductive age, it is impossible to know how many women are affected by adenomyosis. That is because diagnosis of adenomyosis is often difficult. The gold standard tool for diagnosing adenomyosis is by histopathological examination of a womb which has been removed by hysterectomy, which of course is not an option or preferred choice for everyone. In contrast, the gold standard tool for diagnosing endometriosis is a laparoscopy (keyhole surgery), which does not necessitate removal of any organ. Up to 1 in 5 women attending a gynaecology clinic with heavy periods, pelvic pain or infertility, were found to have evidence of adenomyosis on ultrasound scan.
Studies using imaging to diagnose adenomyosis have reported an association between adenomyosis and an increased risk of preterm birth, small for gestational age, and pre-eclampsia among pregnant women who conceive spontaneously. Among women undergoing in vitro fertilisation and intracytoplasmic sperm injection treatment, adenomyosis is associated with a reduced rate of pregnancy and live births as well as an increased risk of miscarriage.
Presentation and Diagnosis
To understand adenomyosis, it is necessary to understand that the uterus has different layers. The innermost layer, which lines the uterine cavity, is called the endometrium. An embryo implants in the cells of the endometrium. The endometrium is what is shed each month when a woman has a period. Moving outward, the next layer is composed of mostly muscle and is called the myometrium. The myometrium can be further divided into an inner layer which is also called the junctional zone and an outer layer. The outermost layer of the uterus is a very thin covering called the serosa. In normal women, the “dividing line” between the endometrium and the junctional zone is clear and distinct and is thin.
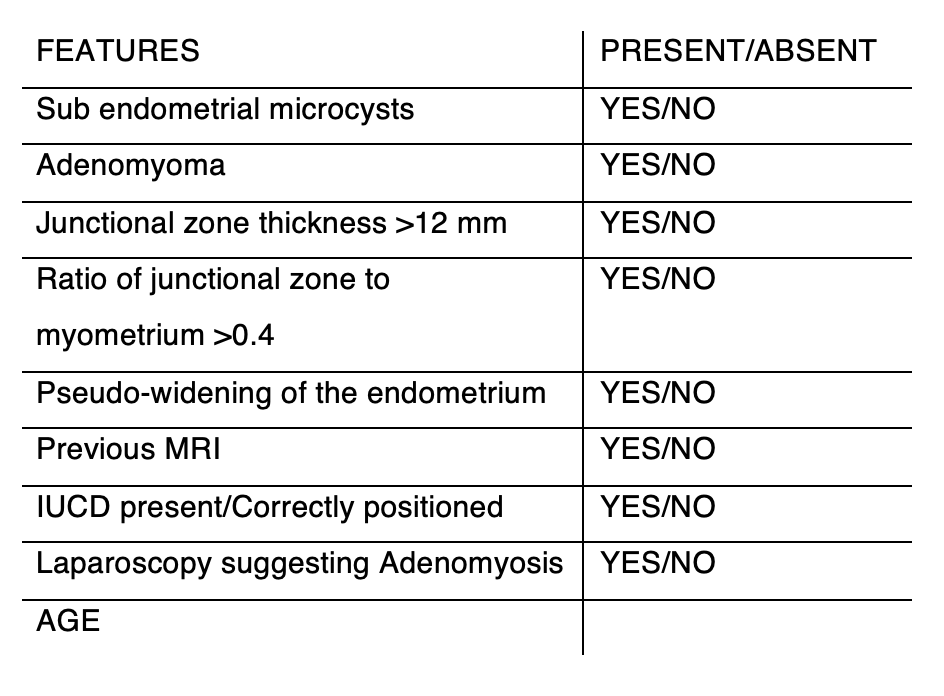
In 80% of cases with histological adenomyosis (hysterectomy specimens), the junctional zone can be seen to be enlarged or thickened on an MRI scan:
- less than 5mm thickening: normal uterus;
- 6-12mm thickening: diagnosis is unclear and could represent developing adenomyosis that not yet reaching diagnostic criteria. The test should be repeated within 12 months;
- more than 12mm: proven adenomyosis.
Another important clue to the diagnosis of adenomyosis, especially in the younger (smaller) uterus, is the ratio of the junctional zone to the myometrium. In the absence of adenomyosis this ratio is less than .4 (40%). Greater than 40% is usually, but not always, also found where the junctional zone thickness is more than 12mm.
In teenage girls with adenomyosis the uterus is not yet matured, so if their junctional zone is in the 5 to 12mm range (strictly not diagnostic), but the ratio of the junctional zone to the myometrium is greater than 40% (in a small uterus), they should be treated as if they have adenomyosis.
This is particularly important where symptoms of endometriosis have led to a laparoscopy but no endometriosis was found. It is usually the case that adenomyosis is present.
Dr Tronc uses this diagnostic table to identify adenomyosis, specifically whether the junctional zone is more than 12mm and whether there is an associated increase in the percentage thickness of the junctional zone amongst other features.
Dr Tronc reports that his choice of scanning techniques for the confirmation of adenomyosis is the MRI scan, not the ultrasound scan, because unless the radiologist is experienced in the diagnosis of early adenomyosis, an ultrasound scan may not give adequate results. In order to get the most accurate diagnosis, women should have the test performed in the “late proliferative” phase, usually on days 10 to 13 of a 28 day cycle. If someone is on the oral contraceptive pill, it seems not to matter when it is done.
It is important to know however that the relationship between JZ thickness and adenomyosis itself is poorly understood, and in about 20% of premenopausal women, the JZ is undefinable on MRI.
Fibrosis is one important feature of adenomyosis. Elastography is a relatively new type of imaging technology that has become available for commercial use. It works by creating images that show how stiff different tissues are. There are two main types: ultrasound elastography (UE) and magnetic resonance elastography (MRE). Ultrasound elastography uses sound waves, while magnetic resonance elastography uses magnetic fields and radio waves.
This technology is similar to the traditional method of feeling for lumps or hardness in a clinical exam (palpation) but offers several advantages. Elastography is less subjective, meaning it doesn’t rely as much on the individual judgment of the clinician. It also doesn’t require as much experience to use, and it provides more precise information about where in the body the stiffness is located.
As of now, the use of MRE in the field of gynecology has been limited. However, ultrasound elastography is becoming more popular in this field. One of the biggest benefits of elastography is that it can detect a wider range of tissue stiffness in adenomyosis compared to other imaging methods like CT scans, standard ultrasounds, and MRI scans.
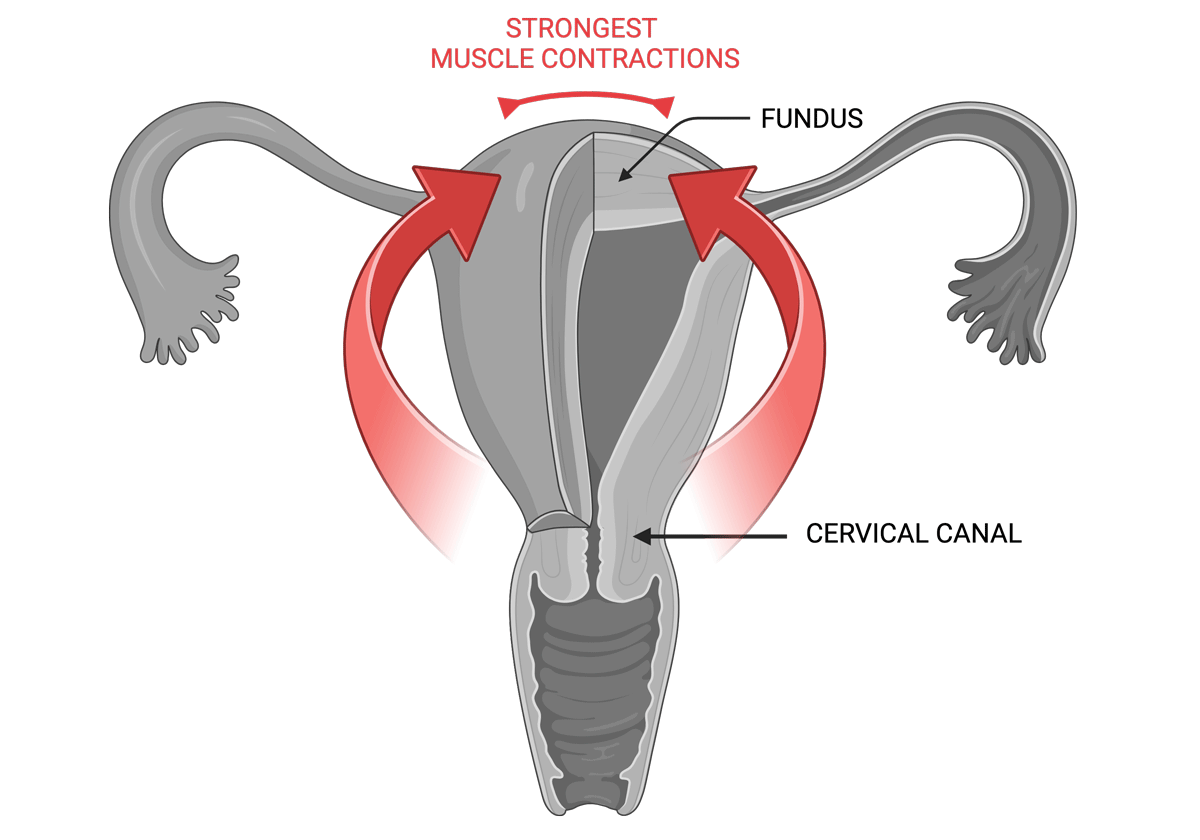
Early adenomyosis usually evolves in the central part of the fundus in the uterus. Even in more advanced cases of adenomyosis the expansion of the junctional zone in MRI often shows concentration of lesions at this location. During menstruation the muscular waves of contraction start in the cervical canal and rapidly move in the fundal direction, exerting their strongest power at the upper level of the uterus, which is where the most trauma will then occur, causing the intense pain of adenomyosis.
Development and Maintenance of Adenomyosis
The most comprehensive theory of of the development of adenomyosis involves the traumatisation of the uterine tissue followed by the initiation of the mechanism of tissue injury and repair (TIAR).
In essence, adenomyotic lesions experience cyclic bleeding and are fundamentally wounds undergoing repeated tissue injury and repair (ReTIAR), which progress to fibrosis, fusing into existing myometrium, and causing an enlarged uterus. The enlarged uterus is likely to result in increased magnitude of uterine contraction, especially when oxytocin receptor (OTR) is overexpressed. In addition, due to the fusion into the myometrium, the uterine contraction is likely to be out of synchronisation, resulting in abnormal and painful muscle contractions.
Wounds may be caused by:
- medical procedures such as dilatation and curettage (D and C), induced abortion, possibly laparoscopy. Wounds from iatrogenic uterine procedures would likely cause intrinsic/internal, but less likely extrinsic/external, adenomyosis simply because of physical proximity. This seems to be supported by published data reporting that internal adenomyosis is associated with a history of uterine procedures. Women with induced abortions are more likely to experience early-stage intrinsic adenomyosis, and women with early-stage extrinsic adenomyosis are more likely to have endometriosis.
- imbalance vaginal microbiome leading to uterine infections
- excess oestrogen causing strong muscle contractions
As long as the endometrial–myometrial interface disruption (EMID) is severe enough, it will cause adenomyosis. Lesional progression can be facilitated by psychological stress, and a history of adverse early life events.
Injury resulting EMID leads to platelet aggregation, inflammation, and hypoxia (low oxygen), causing the release of copious inflammatory cytokines such as IL-1β and growth factors such as TGF-β1, increased local oestrogen production, as well as nerve damage.
Local oestrogen production
Oestrogen is critical to tissue repair, however it can disrupt the normal control that the ovaries have over the uterine muscle movements. As a result, the uterus starts to contract more frequently and intensely than usual, a condition known as hyperperistalsis. This excessive muscular activity can further harm the uterus, creating a cycle where the condition keeps getting worse on its own. Essentially, the ongoing damage leads to more inflammation and hormonal imbalances, which then causes even more damage, continuing the cycle of the disease.
Estradiol’s repair effects are primarily executed through the estrogen receptor-beta (ER2). Research, including animal experiments and studies on various body tissues like astroglia, bladder tissue, fibroblasts, and cartilage, has shown that healing after tissue injury involves locally produced oestrogen.
When tissue is injured, a substance called interleukin-1 activates the cyclooxygenase-2 (COX-2) enzyme, leading to the production of prostaglandin E2 (PGE2). PGE2 then activates the steroidogenic acute regulatory protein (STAR) and the P450 aromatase enzyme. This process facilitates the movement of cholesterol to the inner mitochondrial membrane, where testosterone is produced and subsequently converted into estradiol.
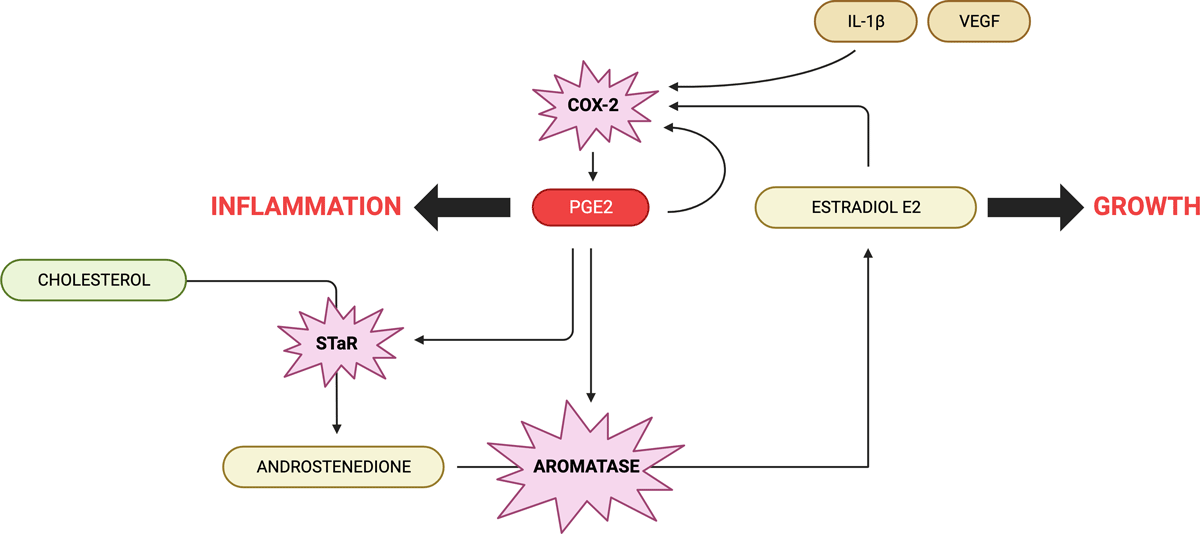
Intriguingly, studies with fibroblasts have shown that even minor physical strain can initiate this healing process.
The similarity of the molecular biology of TIAR in various tissues with that described in endometriosis strongly suggests that this represents the common underlying mechanisms of both diseases.
An increase in nerve fibres and pain
Ectopic endometrial stromal cells, which are cells from the uterus lining found outside their normal location, secrete neurotrophic factors such as NGF, NT-3, TrkB, TXA2, and GDNF, as well as axon guidance molecules like Semaphorin 3E and SLIT/ROBO. These substances stimulate the growth of nerve fibers in the lesions, resulting in an increased density of nerves, known as hyperinnervation.
Additionally, the normal endometrial tissue can promote the growth of both nerves and blood vessels (neuroangiogenesis) through the release of tiny particles called exosomes. This increased nerve presence in the endometrium and the muscle layer of the uterus (myometrium) makes these areas highly sensitive. Therefore, even minor pain signals, changes in pain mediators, or abnormal muscle movements in the uterus can be significantly amplified. These signals are transmitted from the nerves in the uterus to the spinal cord and then to the brain, leading to the perception of pain. The situation is further worsened by the loss of GABAergic inhibition in the nervous system, a mechanism that usually blocks the transfer of pain signals to the brain.
Moreover, with more nerve fibers, especially an elevated density of sensory nerve fibers, there is an increase in the secretion of neuropeptides. However, some neuropeptides, such as substance P and calcitonin gene-related peptide (CGRP), can trigger changes in the ectopic endometrial cells that accelerate the formation of fibrous tissue (fibrosis) in the lesions.
In essence, the interplay of ectopic endometrial cells, increased nerve fibers, and altered neuropeptide levels in adenomyosis synergistically contribute to the pain experienced by women with this condition. This complex interaction involves nerve growth, heightened pain signal transmission, and tissue alterations in the uterus.
Heavy menstrual bleeding, lower PEG2?
In adenomyosis, the tissue becomes stiffer and more fibrous, leading to a decrease in COX-2 activity and lower PGE2 production. The reduction in PGE2 levels disrupts the hypoxia in the endometrium, impairing its ability to repair itself, which contributes to heavy menstrual bleeding. Studies have found that women with adenomyosis who experience heavy menstrual bleeding tend to have greater fibrosis in their lesions, accompanied by lower levels of HIF-1α, COX-2, and other related substances, indicating disrupted prostaglandin signalling and impaired endometrial repair.
Additionally, glycolysis, a process where glucose is broken down to produce energy, plays a crucial role in endometrial repair. The endometrium shifts to glycolysis during repair to meet increased energy demands and reduce oxidative stress, which can damage cells and tissues. However, reduced hypoxia signaling in fibrotic adenomyotic lesions, as seen in decreased levels of HIF-1α, can impair glycolysis. This impairment further disrupts endometrial repair, contributing to heavy menstrual bleeding in adenomyosis.
These findings provide a good explanation as to why different subtypes of adenomyosis have different symptoms. An early study indicated that adenomyosis lesions that penetrate less deeply into the myometrium, the muscular layer of the uterus, are more likely to be associated with heavy menstrual bleeding. This is because lesions closer to the lining of the uterus can more easily affect this area due to their physical proximity, thereby increasing the likelihood of heavy menstrual bleeding as the lesions progress. Additionally, internal adenomyosis, where lesions are within the muscle layer of the uterus, is commonly linked with heavy menstrual bleeding, whereas external adenomyosis, with lesions on the outer part of the uterus, often correlates with deep endometriosis and associated pain.
Heavy menstrual bleeding might further worsen the impairment of endometrial repair. The bleeding could lead to an accumulation of iron in the local area, potentially reducing the growth of endometrial stromal cells by inducing autophagy, a process where cells break down their own components. This could further hinder the endometrium’s ability to repair itself.
A vicious cycle of adenomyosis–pain–stress–lesional progression–more pain
Pain, heavy menstrual bleeding, and infertility can trigger various degrees of anxiety, discomfort, depression, and stress. When stress is chronic or persistent, it activates the Hypothalamic-Pituitary-Adrenal and Sympathetic-Adrenomedullary axes in the body, leading to the release of hormones called catecholamines, including adrenaline and noradrenaline. These hormones act on specific receptors and proteins in the ectopic endometrium (uterine lining cells found outside the uterus), notably adrenergic β2 and cAMP-responsive element-binding protein, accelerating the progression of lesions associated with the condition.
Chronic stress can also diminish the presence of dopamine receptor D2 in the ectopic endometrium. Dopamine is a neurotransmitter involved in mood regulation and other bodily functions. In contrast, positive stress, known as eustress, increases DRD2 presence in the lesions and slows their progression. This finding is consistent with research showing that dopamine or drugs activating DRD2 can hinder stress-related increases in blood vessel formation and cell growth in cancers.
Low dopamine levels can increase prolactin, a hormone important in the reproductive system. High prolactin can disrupt the secretion of gonadotropin-releasing hormone (GnRH), essential for reproductive health, leading to decreased levels of luteinizing hormone and follicle-stimulating hormone, affecting hormone production and fertility.
Prolactin’s elevated levels have been linked to the development of adenomyosis, appearing to encourage the growth and function of cells in the uterus.
In mouse studies, implanting part of the pituitary gland (which produces prolactin) into the uterus induced adenomyosis. Also, higher prolactin levels in the bloodstream were associated with increased adenomyosis incidence. This evidence points to prolactin’s potential role in triggering adenomyosis. Treatment with bromocriptine, a dopamine agonist that reduces prolactin, completely halted adenomyosis development in some studies.
Another aspect of prolactin’s impact is its role in angiogenesis, the formation of new blood vessels. Vascular endothelial growth factor (VEGF) promotes angiogenesis. Dopamine agonists, like cabergoline, hinder VEGF from binding to its receptor on blood vessel cells, thus reducing angiogenesis. Animal studies on endometriosis showed that cabergoline treatment significantly decreased endometriotic lesion size and activity and VEGF production.
A self-perpetuating cycle emerges in adenomyosis, where the condition leads to pain and stress, which then accelerates lesion progression, resulting in more pain. This cycle highlights the intricate interplay between emotional and physical stress and the biological processes in adenomyosis, exacerbating the disease through a combination of hormonal and neurotransmitter effects.
My approach to resolving adenomyosis
Nutrition
- Avoiding gluten, dairy, alcohol, soy and sugar can make a significant difference to reducing pain and inflammation
Supplements
- Vitamin E has been shown to prevent adenomyosis in a study that used Fluoxetine to induce it. Fluoxetine is an antidepressant from SSRI group having effect on reproductive organs by increasing oxidative stress. Administering vitamin E to fluoxetine-induced adenomyosis prevented the rise of prolactin and the development of adenomyosis.
- L-tyrosine increases the production of dopamine and can reduce uterine pain.
- L-arginine can prevent hypoxia and reduce uterine pain and heavy bleeding.
- Agnus castus can reduce prolactin.
- DIM and Myomin can improve the overall balance of oestrogens.
- Quercetin can inhibit the proliferation of ectopic endometrial stromal cells in adenomyosis and reduce their mobility and invasiveness.
Vaginal microbiome swab testing
- I ask all women with suspected adenomyosis to have a vaginal swab test to identify any infections or perturbations in the vaginal microbiome. I have often found the presence of ureaplasma and other infections, a connection not found in research, but echoed by other practitioners.
Takeaways
- Adenomyosis is characterised by a constant pain on the top of the uterus.
- Adenomyotic lesions are fundamentally wounds undergoing repeated tissue injury and repair, which progress to fibrosis, with ensuing greater tissue stiffness, resulting in impaired endometrial repair and eventually causing heavy menstrual bleeding.
- Wounding may be caused by medical uterine procedures, imbalance vaginal microbiome leading to uterine infections and/or excess oestrogen causing strong muscle contractions.
- A high level of prolactin can induce adenomyosis.
- Adenomyosis can be managed by avoiding gluten, dairy, alcohol, soy and sugar.
- Helpful supplements include vitamin E, l-tyrosine, l-arginine, Agnus castus, DIM, Myomin and quercetin.